Mildmay Uganda Research Ethics Committee (MUREC): As stewards of ethical research, we oversee the operations of MUREC, facilitating the review and approval of research protocols. Mildmay Uganda Research Ethics Committee (MUREC) was established in 2014 as a nationally accredited in-house mechanism for reviewing research proposals involving human participants and
their data to ensure they comply to national and international ethical guidelines. MUREC monitors studies before they take off, after they take off and, where necessary, follows up after the end of the research. Our committee comprises experts from diverse backgrounds, ensuring thorough and impartial evaluation of each study to uphold ethical standards. At MRCU, ethical research practices are a top priority. We uphold the highest standards of ethical conduct in research, ensuring the safety, dignity, and rights of all participants involved. Partner with us to advance your research endeavors while prioritizing ethical considerations and human subjects' protection.
Internal Monitoring of Research Studies: Quality and compliance are at the forefront of our internal monitoring efforts. We conduct rigorous monitoring of research studies and clinical trials to ensure adherence to ethical guidelines, regulatory requirements, and best practices. We safeguard the integrity of research outcomes and protect the well-being of participants.
Community Advisory Board Management: Engaging with the community is integral to ethical research. We manage a broad Community Advisory Board (CAB), fostering meaningful dialogue and collaboration between researchers and the community. Our active CAB plays a crucial role in shaping research priorities, ensuring community involvement, and upholding ethical standards in research.
Administrative clearance for research studies: At MRCU, ethical research is our priority. We oversee a rigorous administrative clearance process for all research studies. This ensures that studies accessing patient data or conducting participant interviews adhere to ethical principles and regulatory standards. Partner with us to conduct your research ethically and in compliance with institutional guidelines.
Turnaround time and Efficient Protocol Reviews: At MUREC, we prioritize efficiency to ensure swift and thorough protocol reviews for your research studies. Whether you require fast track reviews, regular reviews, or expedited reviews, our team is committed to delivering timely outcomes without compromising on quality or compliance with international and local regulatory requirements.
With our streamlined review process, you can trust MUREC to provide prompt feedback and guidance, helping you navigate the complexities of research ethics with confidence. Partner with us to ensure your protocols are reviewed promptly and meet the highest standards of quality and compliance.
Contact Mildmay REC today to experience our efficient protocol review services firsthand. Let us help you accelerate your research journey while maintaining the integrity and ethical standards of your studies.
MRCU Monitoring Services
We pride ourselves on offering comprehensive and reliable monitoring services to ensure the success of your research endeavors. With our team of highly trained professionals, you can trust us to monitor your studies with precision and dedication.
Types of Monitoring we conduct:
On-Site Monitoring: Our experienced team conducts thorough on-site visits to oversee every aspect of the study, ensuring compliance with the protocol, Good Clinical Practice (GCP) guidelines and other applicable regulatory requirements. We meticulously review study documentation, data collection procedures, and participant safety protocols to uphold the highest ethical and regulatory standards.
Remote Monitoring: In today's digital age, we understand the importance of flexibility and efficiency. Our remote monitoring services allow us to monitor studies remotely, utilizing advanced technology to review study data and documentation securely. This approach minimizes disruptions to your research while ensuring ongoing compliance and data integrity.
Risk based monitoring (RBM)
At MRCU, we specialize in Risk-Based Monitoring (RBM) to streamline our clinical trials. Our RBM approach focuses on prioritizing resources where they matter most, optimizing efficiency without compromising data quality. Trust us to implement RBM strategies tailored to your study's unique needs, ensuring smooth operations and reliable results.
Why Choose Us:
GCP and HSP Trained: Our team is fully trained in Good Clinical Practice (GCP) and Human Subjects Protection (HSP), ensuring that your research is conducted ethically and in accordance with regulatory requirements at all times.
Tailored Solutions: We understand that every study is unique. That's why we offer personalized monitoring solutions tailored to your specific research needs. Whether you require on-site visits, remote monitoring, or a combination of both, we have the expertise to meet your requirements.
Commitment to Excellence: With a proven track record of excellence, we are committed to delivering superior monitoring services that exceed your expectations. Our attention to detail, professionalism, and dedication to quality set us apart as a trusted partner in research.
At MRCU, we offer a full spectrum of site monitoring visits, ensuring thorough oversight and compliance throughout the lifecycle of your research studies. From pre-site assessments to closeout visits, our dedicated team is committed to upholding the highest standards of quality and integrity in all research conducted under our auspices.
At MRCU, we are committed to promoting ethical research practices and protecting the rights and welfare of research participants. Investigators seeking administrative clearance for their research studies can rely on the expertise and support of our Bioethics Department to ensure ethical integrity and regulatory compliance.
How To Submit a Protocol for Ethical Review
Are you ready to submit your research protocol for ethical review? Here's how to get started!
Step 1: Register on NRIMS
All applications are submitted online via the National Research Information Management System (NRIMS). First-time users must create an account at https://nrims.uncst.go.ug
Upon registration, you’ll receive your username, created password, and an account activation link via email.
Tip: Check your spam/junk folder if you don’t see the email right away.
Activate your account by clicking the link in the email. Once activated, log in at the NRIMS homepage and proceed to submit your application.
Step 2: Submit Your Protocol
Log in to NRIMS using your credentials.
Click on the ‘Submit New Protocol’ tab.
Respond to the question, “Does the study involve human participants?” (Choose ‘Yes’ if applicable).
When prompted to “Choose REC”, select “Mildmay Uganda REC.”
Step 3: Prepare Your Documents
You’ll need the following files (all in PDF):
- A Cover letter (A request letter addressed to the Chairperson MUREC to review the protocol)
- Passport photo of the applicant (white background).
- Ethical approval from the parent institution (where applicable)
- Commitment and support letters from collaborating sites (where applicable)
- CVs of Principal investigator, Co-investigators and other investigators not exceeding 4 pages but highlighting core competencies in the intended area of study.
- Evidence that the researchers are qualified for the study. Valid professional practicing licenses
- Valid human subjects’ protection certificate and or Good Clinical Practice (GCP)
- Consent form/assent form should follow the UNCST format. Where applicable with translations in local languages for the study participant)
- Protocol document which includes: a. Full protocol including information dissemination plan b. Consent and assent forms (where applicable) with translations in local languages for the study participants c. Final data collection instruments (Where applicable with translations in local languages for the study participants) d. Work plan e. Budget
- Payment slip.
- Brief community engagement plan (mandatory field in the system).
- Templates for informed consent forms, community engagement plans, and COVID-19 risk management plans are available from the MUREC Administrator.
Step 4: Approval and Fees
For academic proposals (PhD, Masters, Undergraduate), ensure departmental or faculty approval and relevant signatures before submission.
Important Information for Applicants
To ensure timely review of your study, kindly note the following:
- Regular Reviews: Submit your application at least 10 working days before the last Friday of the month (when review meetings are held).
- Fast-Track Reviews: These are processed immediately upon submission, with feedback provided within 7 working days.
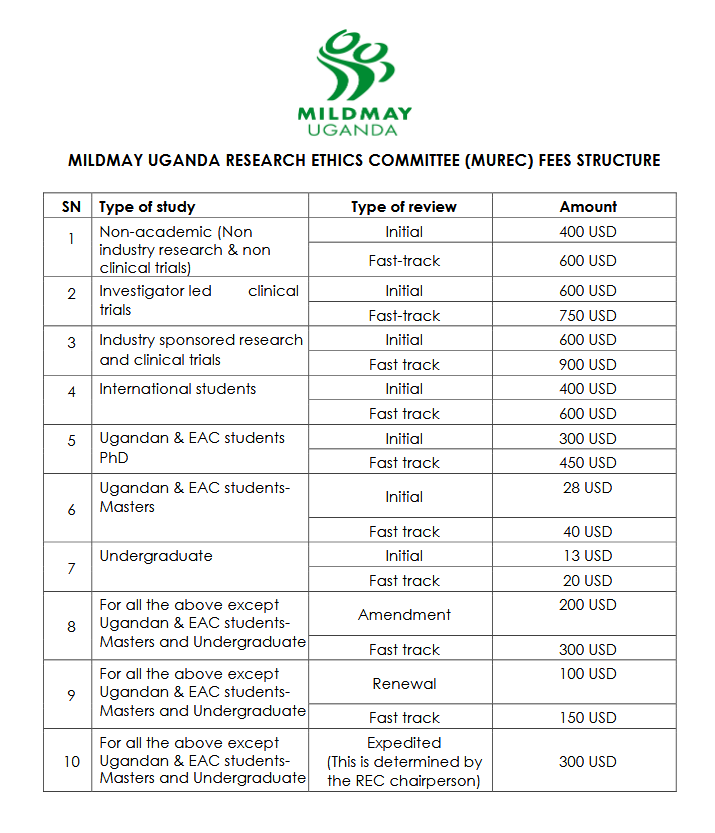
- Review Fees: Fees are standardized across Uganda’s Research Ethics Committees (RECs).
For assistance with payment, administrative clearance, or ethical research support, please reach out to:
Ms. Racheal Dedibo
MUREC Administrator
📞 +256 701 472 492 | +256 392 174 236
Office Location
Mildmay Uganda Research Ethics Committee (MUREC) Secretariat
International House (MRCU Building)
Entebbe Rd, Lweza-Nazziba Hill,
Wakiso, Uganda
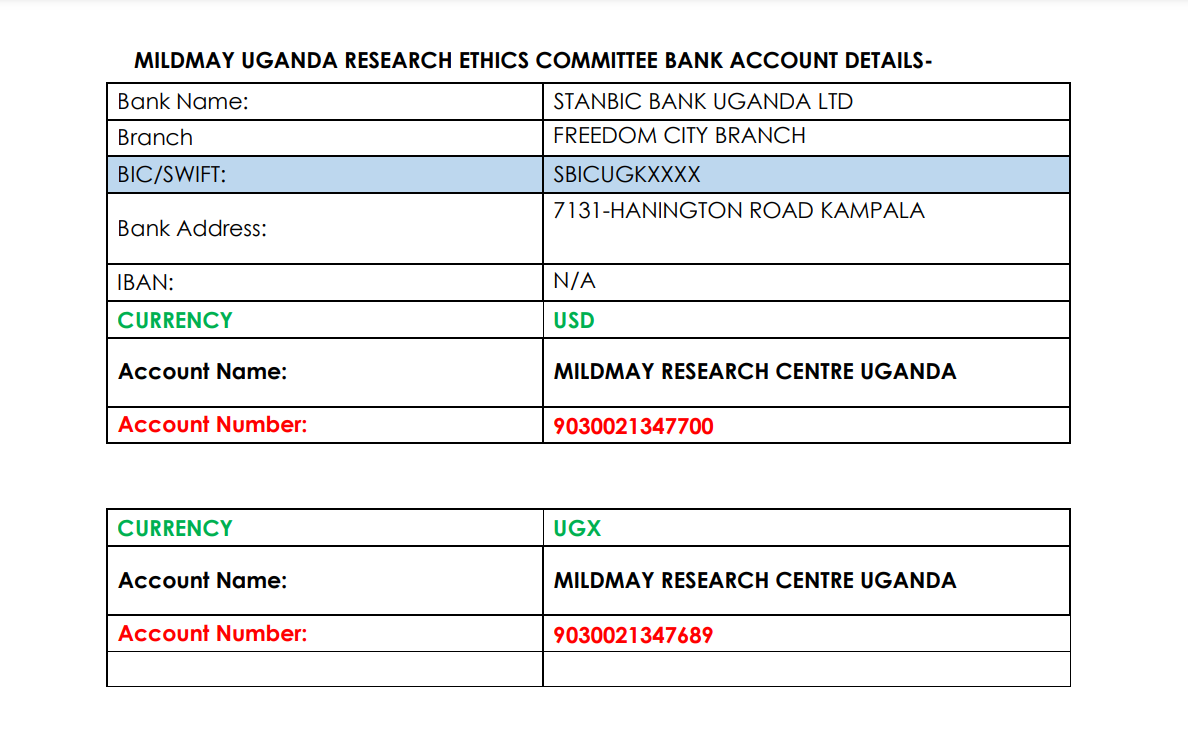
Payments are to be made through the Bank.